
Gypsum as a Sulfur Source is Smart for your Soil
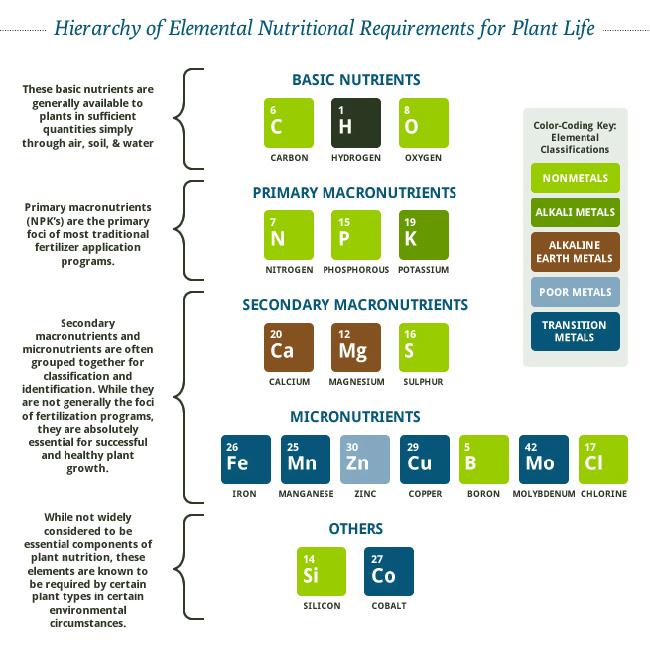
Gypsum (21% Calcium, 17% Sulfur)
• Neutral pH won't further acidify your soil
• Low salt index so it's safe for the soil
• Improves soil structure by adding calcium
• Slower sulfur release for more yield
4 Reasons Why:
Gypsum has a neutral pH, so it won’t further acidify the soil, unlike ammonium or elemental sulfur sources. Last winter we took a look at soil sample results that we had data from the last 10+ years to see what the trend in soil pH was, the result was clear.
why we think Gypsum is well suited as a Sulfur source in Central AB: Gypsum adds calcium which will help improve the structure of your soil. The calcium in Gypsum will help to deflocculate your soil, improving water infiltration & allow more root growth.As soil gets too high concentration of Na, it becomes flocculated or tightly bound because Na is a '+' ion & clay is '-' charge. Na has a small particle size & so the soil becomes tightly bound with little pore space. Sodium Adsorption Ratio can tell us if this is a concern.
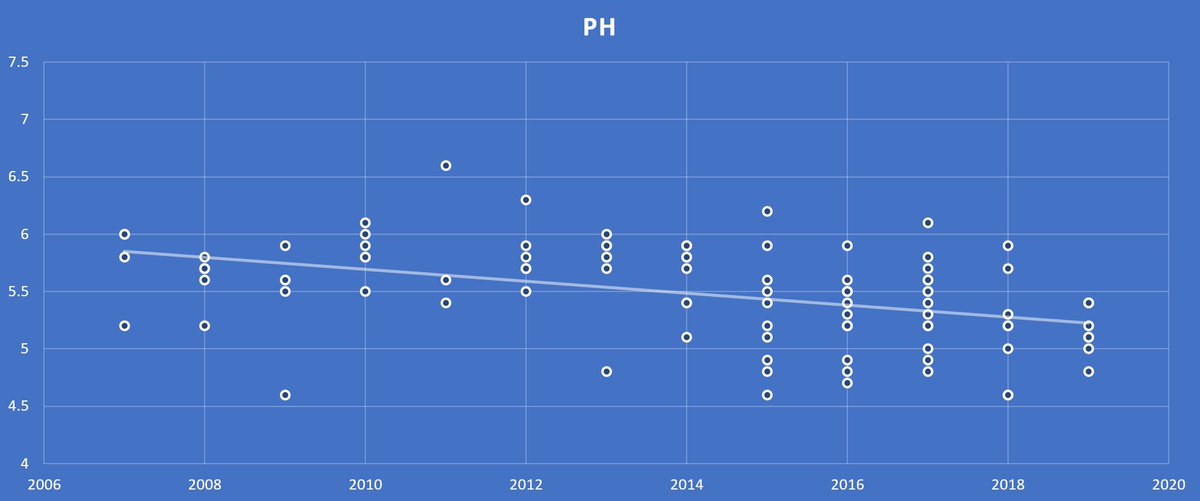
On fields we've been soil sampling consistently for several years, we have seen avg pH drop from 6.0 – 5.5. That drop in pH means that soil has lost 12% efficiency of Nitrogen (89% efficient at 6.0, 77% at 5.5). Phos is 52% at 6.0, 48% at 5.5. K is 100% at 6.0, 77% at 5.5.
This website from Cornell University shows the CaC03 equivalent needed to offset the application of ammonia fertilizers. For every 25 # of S applied as Ammonia Sulfate, 135 lbs of Lime would need to be applied to the soil to maintain pH.
https://nrcca.cals.cornell.edu/nutrient/CA5/CA0537.php
Gypsum won't raise soil pH, Lime is needed for that. However, using Gypsum as your sulfur source won't decrease pH like ammonia or elemental sulfur sources will.
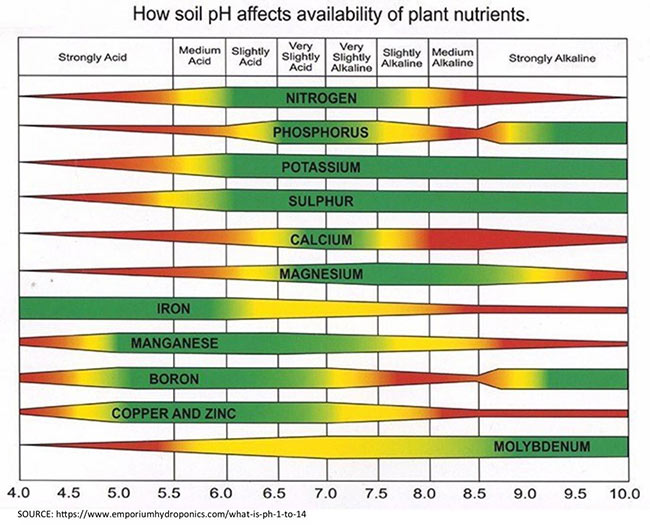
Gypsum won't raise soil pH, Lime is needed for that. However, using Gypsum as your sulfur source won't decrease pH like ammonia or elemental sulfur sources will.

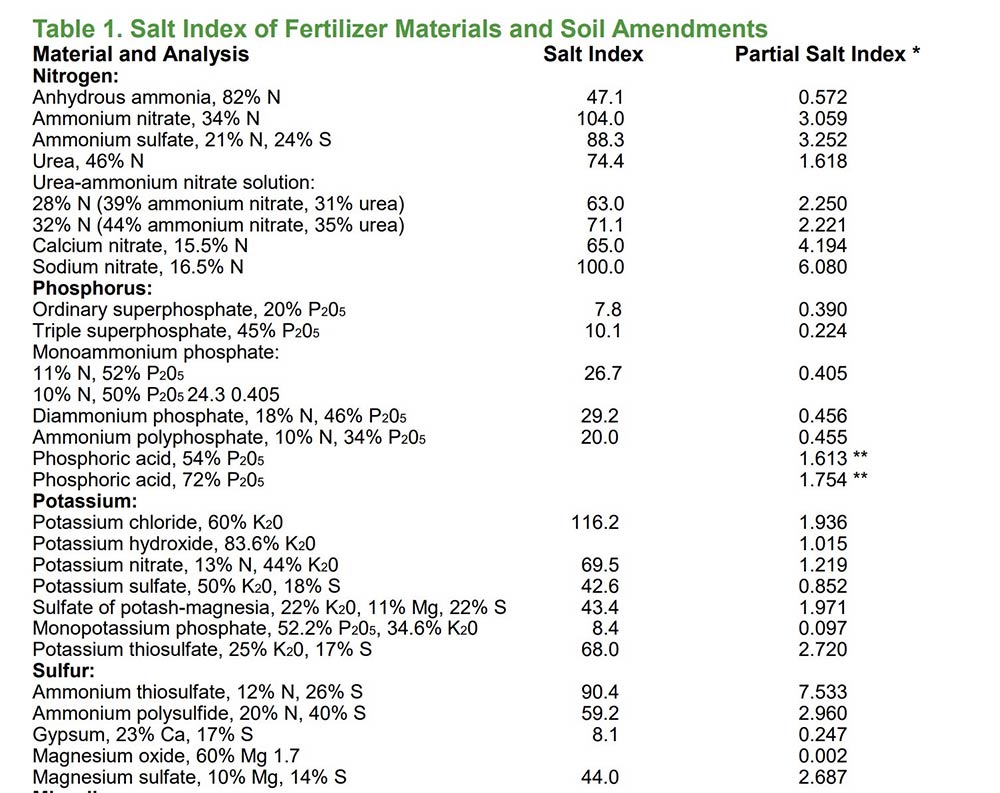
Why we think that Gypsum is advantageous as a sulfur source: Gypsum has a low salt index, so it’s safer for the soil and seedlings, especially canola (& peas). Salt index is the measure of a fertilizers salt effect compared to sodium nitrate (salt index of 100).
Gypsum has a salt index of 8.1, while ammonium sulfate is 88.3. Therefore, the application of Gypsum as a Sulfur source will help to reduce the amount of salt being applied to soil. Gypsum will also help to remediate high salt concentrations.
 Too high of salts in the soil can affect the plants ability to take up nutrients & water. If salt content of soil gets too high, it can actually pull water from roots. Read the effect of Salinity on Canola Growth & Yield in the Canola Encyclopedia.
Too high of salts in the soil can affect the plants ability to take up nutrients & water. If salt content of soil gets too high, it can actually pull water from roots. Read the effect of Salinity on Canola Growth & Yield in the Canola Encyclopedia.
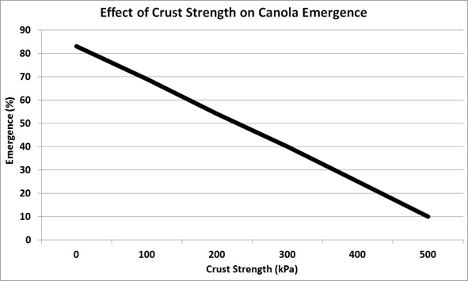 Canola Council Effect Of Soil Characteristics
Salts from fertilizers is relatively low compared to the entire mass of soil, but when applied in the top few inches it can affect germ & growth. By using Gypsum as the Sulfur, we're removing a lot of salts that will give seedlings a better chance of emerging & thriving.
Canola Council Effect Of Soil Characteristics
Salts from fertilizers is relatively low compared to the entire mass of soil, but when applied in the top few inches it can affect germ & growth. By using Gypsum as the Sulfur, we're removing a lot of salts that will give seedlings a better chance of emerging & thriving.
Why we think Gypsum is well suited as a Sulfur source in Central AB: Gypsum adds calcium which will help improve the structure of your soil. The calcium in Gypsum will help to deflocculate your soil, improving water infiltration & allow more root growth.
As soil gets too high concentration of Na, it becomes flocculated or tightly bound because Na is a '+' ion & clay is '-' charge. Na has a small particle size & so the soil becomes tightly bound with little pore space. Sodium Adsorption Ratio can tell us if this is a concern.
This video explains & shows deflocculation well (2:35 talks about flocculated soils): https://youtube.com/watch?v=tLaJawbMrT0…
This video explains S.A.R: https://youtube/83qEk0sH8qo
This video from @AgPhDMedia talks about benefits of gypsum & calcium: https://youtu.be/FKZ4M1C60xI
Calcium will replace Na on the soil particle, Ca has a large particle size & so pore spacing will then improve (think about the difference of aerating peas or canola in a bin). This allows water, roots, oxygen, etc to move through the soil profile, improving soil health.
Calcium is also needed by the plant, it's considered a macro-nutrient & is needed in large quantities. It will help considerably with cell-wall structure & is being researched as to its ability to help the canola plant fight off clubroot & blackleg.
Depending on Na levels of soil & other factors, it may take 1000's of pounds of Gypsum to remedy. However, by using Gypsum as a sulfur source, we're supplying some Ca to start the remediation
Why we think Gypsum as a Sulfur Source is a good fit for Central Alberta. Gypsum has a slower sulfur release than Ammonium Sulfate, so there will be more Sulfur available when the canola plant needs it. Especially if fall applied.
For sulfur to be plant available, it needs to be in the Sulfate form. However, Sulfate is prone to leaching & quite mobile in the soil. Amm. Sulfate rapidly dissolves with adequate soil moisture & leaching can begin within 5-7 days, depending on soil temp (cooler = slower)
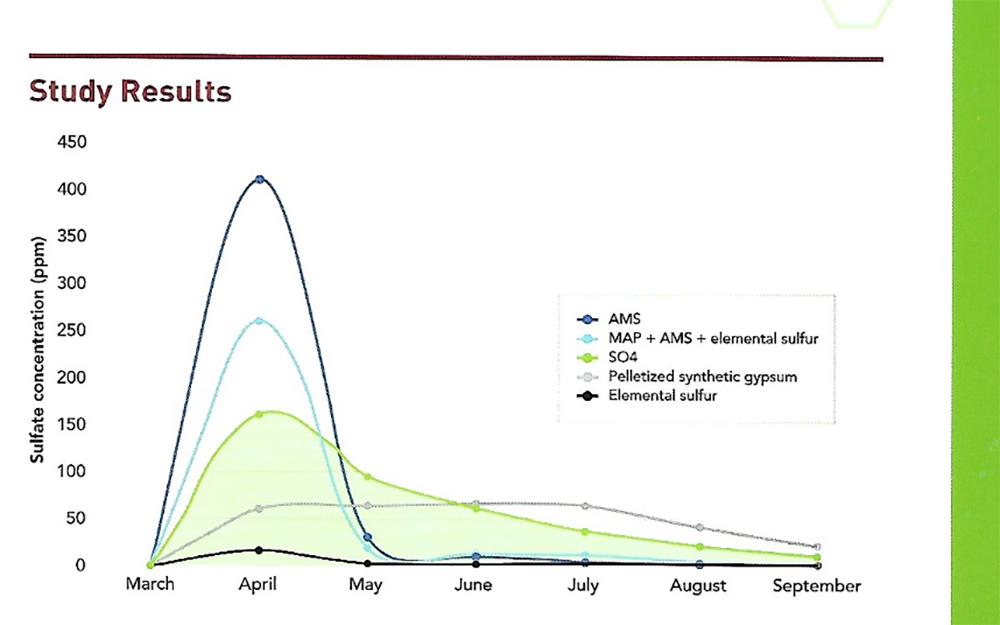
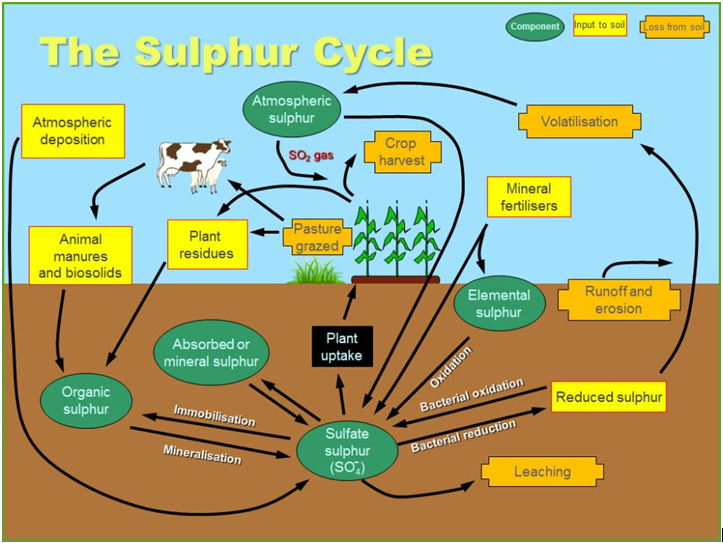 As Gypsum is not as soluble in water as Amm. Sulfate, the breakdown to leachable form of S is not as fast. Gypsum will release a moderate amount of S shortly after application & then continuously release Sulfur through the season. See the pic, where S04 is a brand of Gypsum.
As Gypsum is not as soluble in water as Amm. Sulfate, the breakdown to leachable form of S is not as fast. Gypsum will release a moderate amount of S shortly after application & then continuously release Sulfur through the season. See the pic, where S04 is a brand of Gypsum.
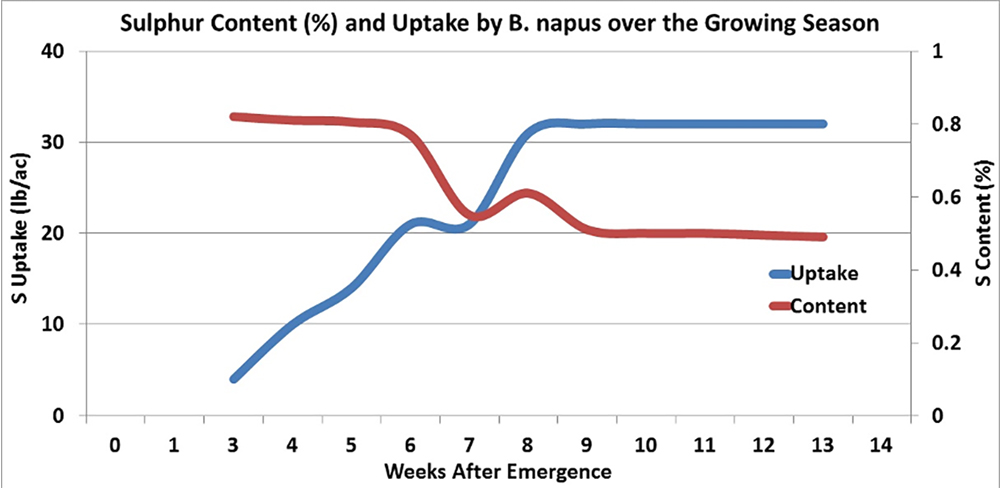
 This means that the release of Sulfur is well matched to canola’s uptake of Sulfur, which is moderate through to cabbaging, then plateaus until podding begins, podding is where it’s crucial to have sulfate availability to ensure max yield potential.
This means that the release of Sulfur is well matched to canola’s uptake of Sulfur, which is moderate through to cabbaging, then plateaus until podding begins, podding is where it’s crucial to have sulfate availability to ensure max yield potential.
 For fall applied Sulfur programs, especially in wet soil conditions, it wouldn't be unreasonable to think that 20+% of S applied as AMS would be unavailable when canola needs it next spring. Thus, using gypsum we can apply less lbs of S &/or achieve higher yield potential.
For fall applied Sulfur programs, especially in wet soil conditions, it wouldn't be unreasonable to think that 20+% of S applied as AMS would be unavailable when canola needs it next spring. Thus, using gypsum we can apply less lbs of S &/or achieve higher yield potential.
